The Notch receptor
Understanding structural and functional aspects of Notch-ligand interactions:
The Notch receptor mediates a key short-range signal which is used in many developmental contexts to refine cell fate choices and define boundaries between different cell types. Notch also functions in the adult organism, where it plays a major role in tissue homeostasis through the maintenance and/or the fate choices of tissue stem cells. Genetic diseases affecting Notch pathway components give rise to a wide range of congenital and adult-onset disorders, consistent with the role of Notch in a core signalling pathway, Furthermore, dysregulation of Notch has also been found to contribute to many types of human cancer, making Notch signalling a strong candidate for therapeutic intervention. We are using a range of molecular and cellular strategies to understand:
i) the cell-surface organisation of Notch and its ligands,
ii) how Notch- ligand complexes form and subsequently lead to activation
iii) how this process is regulated
This interdisciplinary work involves the following collaborations (Lea-X-ray crystallography; Redfield-NMR; Baron-Drosophila model organism; Haltiwanger-glycosylation; Harris/Banham-therapeutic antibodies)
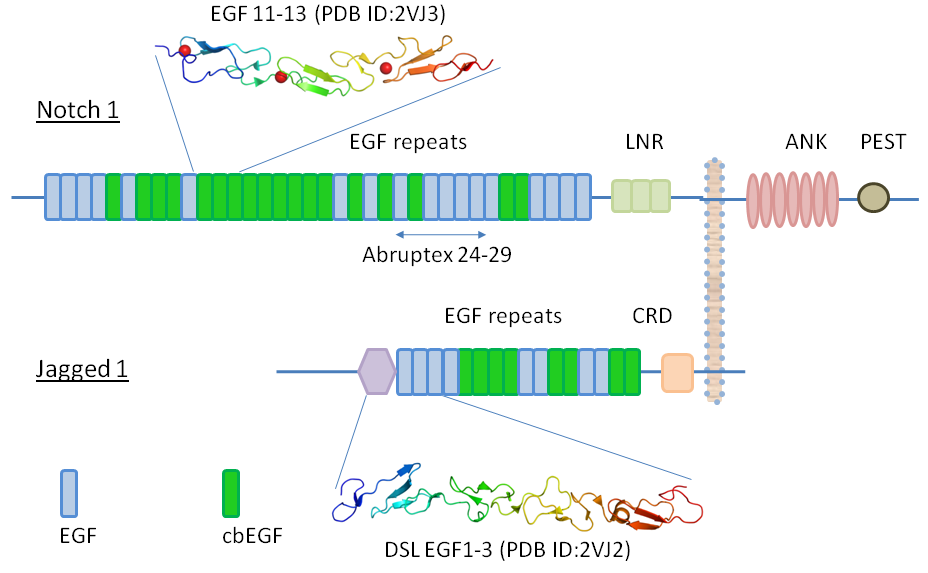
Notch structure and domain organisation
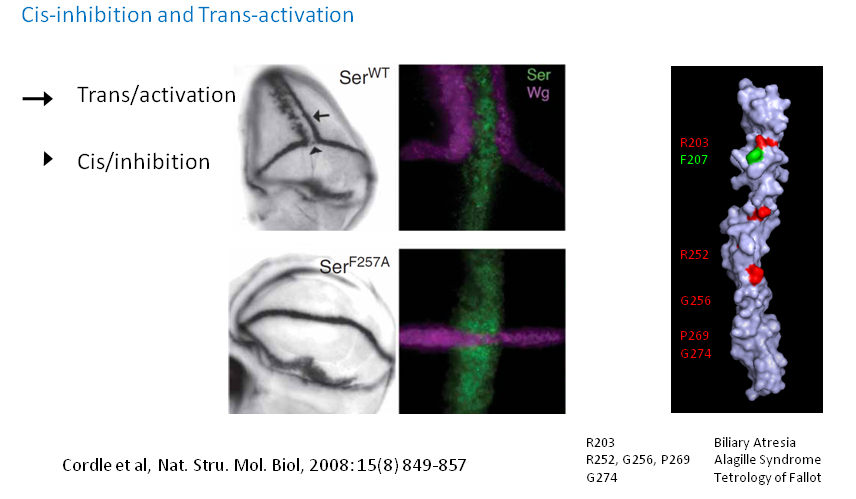
Cis and trans interactions
Notch receptors are activated by ligands on neighbouring cells (trans-activation) but can also be inhibited by ligands when expressed on the same cell surface (cis-inhibition). Our earlier work showed that a similar face of Jagged-1 (DSL domain) is involved in both interactions. Though the region involved in trans-activation is identified in the Notch receptor (EGF11-12 domains) it is not yet established which region is involved in cis-inhibition. There is a possibility that trans- and cis-interactions occur in anti-parallel and parallel orientations using same faces on both proteins, though other sites of interaction cannot be ruled out. We are devising biochemical and biophysical methods to clearly understand the molecular interactions between receptor and ligands leading to these two regulatory interactions.
Within the EGF11-12 region, there are several non-structural residues that are highly conserved between different species. We have mutated several residues within the ligand binding region, and tested the ability of Notch mutants to bind to ligand using a flow-cytometry assay. In collaboration with Dr Martin Baron, University of Manchester, we have also made corresponding site-directed mutants in Drosophila Notch and tested their effects in cell aggregation assays. We have also tested the effects of specific sugar modifications (in collaboration with Professor Robert Haltiwanger, StonyBrook) within the ligand-binding region of the Notch receptor on binding to the two separate classes of Notch ligands. We hope to identify the structural basis for ligand binding in this region,and how this can be differentially regulated by glycosylation.
Structural organisation of the Notch receptor
Decades of research in the field has given good insight into the downstream consequences of Notch signaling but there is very little knowledge about the cell-surface organization of Notch receptor and how the interaction with ligand leads to receptor activation. Since Notch is a large glycoprotein made up of many disulphide-rich domains, working on the whole molecule is difficult. We have focussed on dissecting the receptor into smaller fragments and are using a combination of high resolution structural methods such as NMR (with Dr Christina Redfield) and X-ray crystallography (Professor Susan Lea) to give new insights into the structural organisation and dynamics of Notch.
Abruptex region
The region from EGF24-29 of Drosophila melanogaster Notch receptor is named Abruptex (Ax) region. There are three classes of Ax mutations: lethal, N suppressor, and N enhancer, observed in Drosophila. The combinations amongst different mutant classes, and with a Notch null mutation, generate an interesting spectrum of phenotypes in the flies for which there is no known molecular basis. It also has been reported in an in vitro study that the Ax region competes with the Notch ligand Delta for the ligand binding region. We are investigating how the Ax region functions at molecular level and results in the different phenotypes.
Towards therapeutic applications
The Notch pathway has an essential role in vascular development and patterning and is also implicated in regulation of blood vessel sprouting and branching in pathological angiogenesis. Notch receptors and ligands are often expressed by tumour cells of different origin in which they play an oncogenic function. Modulation of the pathway may therefore have significant therapeutic potential. In collaboration with Professor Adrian Harris and Dr Alison Banham we aim to generate and pre-clinically test novel therapeutic monoclonal antibody reagents that could have widespread applicability for the treatment of cancer.