 Degradation of Misfolded Glycoproteins in the ER
Degradation of Misfolded Glycoproteins in the ERDr Terry D. Butters, MPhil, PhD
- contact details
Reader in Glycobiology Institute
The use of small molecule inhibitors
to enzymes involved in glycoconjugate metabolism is being explored in a number
of therapeutic applications. Most of the projects aim to understand the
biochemical mechanism for action of inhibitors in a cellular environment.
These projects are expected to:
i) contribute to the design of improved therapeutics
ii) dissect the pathways of glycoconjugate metabolism in cells and in vivo.
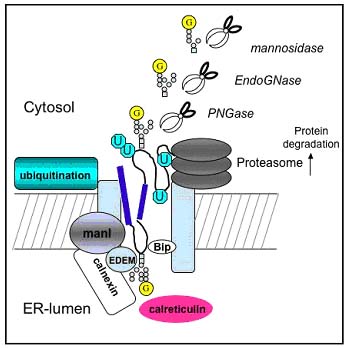
1. Analysis of early events in the endoplasmic reticulum (ER) that control N-glycoprotein biosynthesis and regulate protein-folding pathways.
When misfolded glycoproteins are removed from the lumen of the ER and targeted for proteolytic degradation, the analysis of free N-linked oligosaccharides that are generated offer a simple marker for ER-regulated protein misfolding. The isolation and characterisation of these free oligosaccharides by HPLC reveals pathways for clearance and can be used to quantitatively measure the effects of inhibitors on ER-resident glycoprotein processing -glucosidases.
 Degradation of Misfolded Glycoproteins in the ER
Degradation of Misfolded Glycoproteins in the ER
The design and synthesis of novel -glucosidase inhibitors that gain entry to the ER and disrupt protein-folding pathways at low (sub µM) concentrations are evaluated for inhibition of viral (HIV, hepatitis) infectivity and these discoveries lead to improvements in anti-viral strategies.
Collaborators: Professor Tim Block, Drexel University; Professor Paul Wentworth, Scripps Institute; Professor George Fleet, Oxford; Dr Nicole Zitzmann, Oxford; Dr Antony Fairbanks, Oxford; Dr Ben Davis, Oxford.
2. Novel imino sugars for chaperon-mediated therapy for glycolipid lysosomal storage disorders (GLSD).
In many human GLSD, mutations in the gene result in incorrectly folded glycosidase protein that fails to traffic to the lysosome leading to decreased enzyme activity and substrate accumulation. Specific imino sugar inhibitors to lysosomal enzyme can assist protein folding if delivered at low concentrations during translation in the ER, improving catalytic efficiency in the lysosome. Strategies that aim to understand mechanism of action, increase ER access and the design and synthesis of selective inhibitors offer a novel and disease specific treatment for the GLSD.
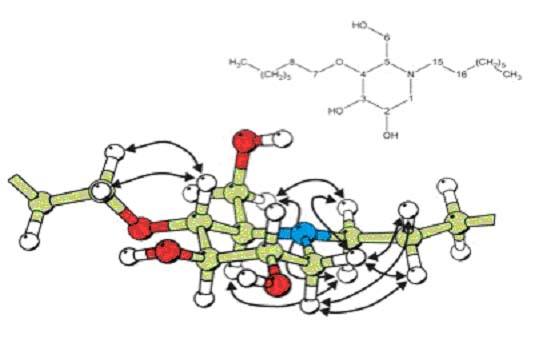 Novel ceramide glucosyltransferase inhibitors (Boucheron et al)
Novel ceramide glucosyltransferase inhibitors (Boucheron et al)
The target enzymes here are beta-glucocerebrosidase (Gaucher disease), alpha-galactosidase (Fabry disease) and hexosaminidases (Tay-Sachs and Sandhoff disease).
Collaborators: Professor Paul Wentworth, Scripps Institute; Professor George Fleet, Oxford.
3. Imino sugars and disease pathology.
The utility of imino sugars to modulate both protein and lipid glycosylation
has therapeutic benefit and a number of projects aim to explore this potential:
(i) novel inhibitors for substrate reduction therapy (SRT) to treat the
glycoshingolipidoses;
(ii) development of in vitro lysosomal storage disease cells with authentic
glycoconjugate products that mimic human lysosomal storage disease phenotype.
Inhibitor manipulation of hexosaminidase activity to generate Sandhoff disease
in cell of different lineage are being used to explore the mechanism of
pathogenesis at the single cell level;
(iii) route to the synthesis of novel hexosaminidase inhibitors are being
sought to offer therapeutic intervention in macrophage recognition of tumour
cells;
(iv) the inhibition of plasma membrane glycolipids to probe their role
in:
- ABC protein transporter activity, MDR for example
- CD1 glycolipid antigen presentation and NKT cell recognition
- Membrane microdomain function.
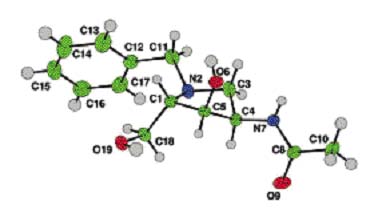 Novel Hexosaminidase inhibitors (Harding et al)
Novel Hexosaminidase inhibitors (Harding et al)
Collaborators: Professor George Fleet, Oxford; Dr Yves Bleriot, Paris; Professor Olivier Martin and Dr Philippe Compain, Orleans; Dr Richard Callaghan, Oxford; Dr Fedor Berditchevski and Dr Elena Odintsova, Birmingham;. Professor Anthony Magee, London;. Professor Vincenzo Cerundolo, Oxford; Dr Mikael Bols, Aarhus.
Selected recent publications :
Inhibitors of Glycosphingolipid Biosynthesis: Application to Lysosomal
Storage Disorders.
Butters, T.D., Dwek, R.A. and Platt, F.M. (2000) Chem Rev 100, 4683-4696.
Novel oral treatment of Gaucher's Disease with N-butyldeoxynojirimycin
(OGT-918) to decrease substrate biosynthesis.
Cox, T., Lachmann, R., Hollak, C., Aerts, J., van Weely, S., Hrebicek, M.,
Platt, F., Butters, T., Dwek, R., Moyses, C., Gow, I., Elstein, D. and Zimran,
A. (2000) The Lancet, 355, 1481-1485.
Study of the Mechanism of the Antiviral Action of Iminosugar Derivatives
Against Bovine Viral Diarrhea Virus.
Durantel, D., Branza-Nichita, N., Carrouée-Durantel, S., Butters,
T.D., Dwek, R.A. and Zitzmann, N. (2001) J Virol 75, 8987-8998.
Targeting Glycosylation as a Therapeutic Approach.
Dwek, R.A., Butters, T.D., Platt, F.M. and Zitzmann, N. (2002) Nature Rev
Drug Disc 1, 65-75.
Analysis of fluorescently labelled glycosphingolipid-derived oligosaccharides
following ceramide glycanase digestion and anthranilic acid labelling.
Neville, D.C.A., Coquard, V., Priestman, D.A., te Vruchte, D.J.M., Sillence,
D.J., Dwek, R.A., Platt, F.M. and Butters, T.D. (2004) Anal Biochem 331,
275-282.
Cellular effects of deoxynojirimycin analogues. Uptake, retention and inhibition
of glycosphingolipid biosynthesis.
Mellor, H.R., Neville, D.C.A., Harvey, D.J., Platt, F.M., Dwek, R.A. and
Butters, T.D. (2004) Biochem J 381, 861-866.
Cellular effects of deoxynojirimycin analogues. Inhibition of N-linked
oligosaccharide processing and generation of free glucosylated oligosaccharides.
Mellor, H.R., Neville, D.C.A., Harvey, D.J., Platt, F.M., Dwek, R.A. and
Butters, T.D. (2004) Biochem J 381, 867-875.
Inhibition of glucosylceramide synthase does not reverse drug resistance
in cancer cells.
Norris Cervetto, E., Callaghan, R., Platt, F.M., Dwek, R.A. and Butters,
T.D. (2004) J Biol Chem 279, 40412-40418.
Design and synthesis of iminosugar-based inhibitors of glucosylceramide
synthase: the search for new therapeutic agents against Gaucher disease.
Boucheron, C., Desvergnes, V., Compain, P., Martin, O.R., Lavi, A., Mackeen,
M., Wormald, M.R., Dwek, R.A. and Butters, T.D. (2005) Tetrahedron Asymmetry,
16, 1747-1756.
2-Acetamido-N-benzyl-1,4-imino-1,2,4-trideoxy-L-arabinitol 0.33-hydrate.
Harding, C.C., Rountree, J.S.S., Watkin, D.J., Cowley, A.R., Butters, T.D.,
Wormald, M.R., Dwek, R.A. and Fleet, G.W.J. (2005), Acta Cryst., E61, o1683-o1685.
Imino Sugar Inhibitors for the Glycosphingolipidoses.
Butters, T.D., Dwek, R.A. and Platt, F.M. (2005) Glycobiology 15, 43R-52R.