References
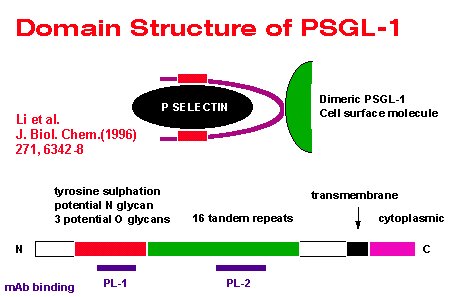
They have shown that for the interaction with P-selectin the PSGL 1 molecule must be dimeric, express the sialyl-Lex epitope and also carry orther post-translational modifications. They have proposed the following domain structure for the molecule

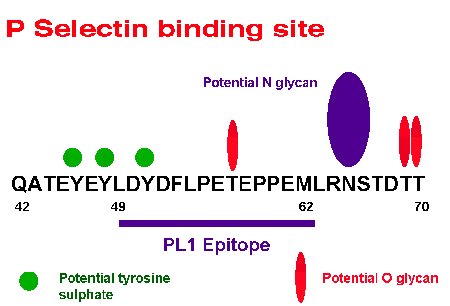
In particular the binding of monoclonal antibody PL1 which specifically blocks interaction with P selectin has been shown to be dependent on tyrosine sulphation as well as an O-glycan with the sialyl-Lex epitope. The predicted binding site on PSGL-1 determined by their wrk is shown below.
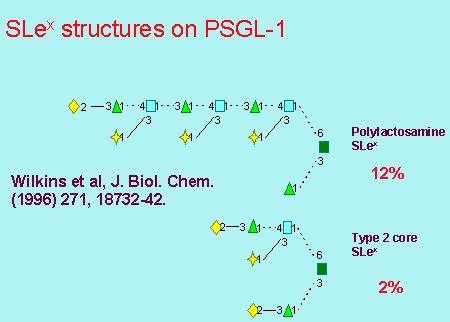
The group has also studied the O-glycans present on the native molecule and have found the following structures which express the sialyl-Lex epitope