|
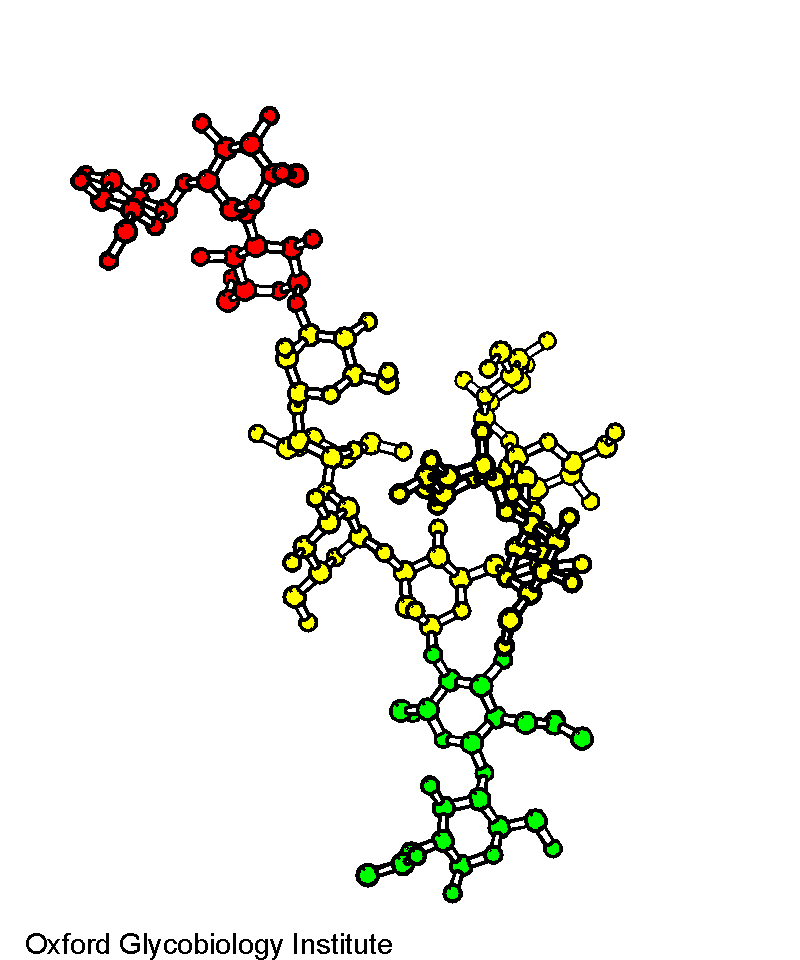
Glc3Man9GlcNAc2
Average solution structure determined by NMR. Glucose resdiues are in red,
mannose residues are in yellow and N-acetyl-glucosamine residues are in
green.
The solution NMR structure of glucosylated N-glycans
involved in the early stages of glycoprotein biosynthesis and folding.
A.J. Petrescu, T.D. Butters, G. Reinkensmeier, S.
Petrescu, F.M. Platt, R.A. Dwek and M.R. Wormald (1997) EMBO J., 16, 4302-4310.
|
|
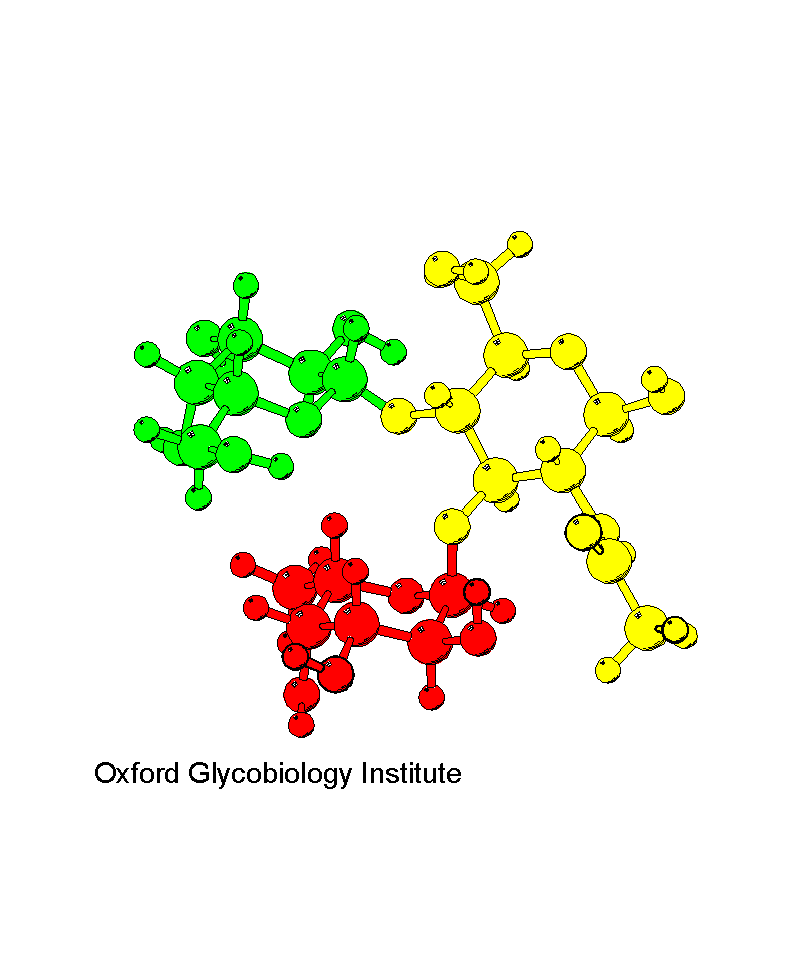
Lewis X epitope
Structure determined by NMR and molecular modelling. This is an example
of a rigid oligosaccharide structure, resulting from the stacking of the
Fuc (red) and Gal (green) rings.
The solution conformation of the Lex group.
M.R. Wormald, C.J. Edge and R.A. Dwek (1991) Biochem.
Biophys. Res. Comm., 180, 1214-1221.
The systematic use of negative nuclear Overhauser
constraints in the determination of oligosaccharide conformations: application
to sialyl-Lewis X.
M.R. Wormald and C.J. Edge (1993) Carbohyd. Res.,
246, 337-344.
|
|
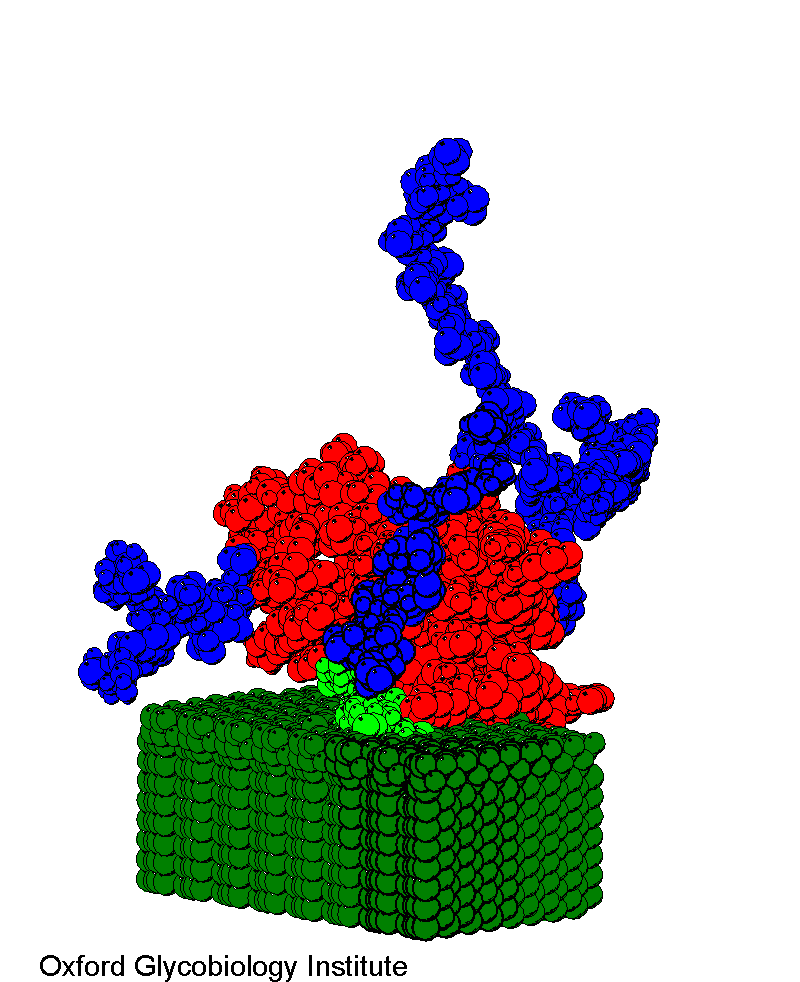
Thy 1
Model based on a standard immunoglobulin fold for the protein and the glycosylation
analysis. The GPI anchor (light green) is inserted into a simple model membrane
(dark green).
Complete structure of the glycosylphosphatidylinositol
membrane anchor of rat brain Thy-1 glycoprotein.
S.W. Homans, M.A.J. Ferguson, R.A. Dwek, T.W. Rademacher,
R. Anand and A.F. Williams (1988) Nature, 333, 269-272.
Dropping anchor with the lipophosphoglycans.
T.W. Rademacher, C.J. Edge and R.A. Dwek. (1991) Current
Biology, 1, 41-42.
Comparative analysis of the N-glycans of rat, mouse
and human Thy-1. Site-Specific Oligosaccharide Patterns of Neural Thy-1, a
Member of the Immunoglobin Superfamily.
T.W. Rademacher, R.B. Parekh, D.R. Wing, A.C. Willis,
A.N. Barclay, R. Dalchau, J.W. Fabre, R.A. Dwek and A.F. Williams (1993)
Glycobiology, 3, 339-348.
|
|
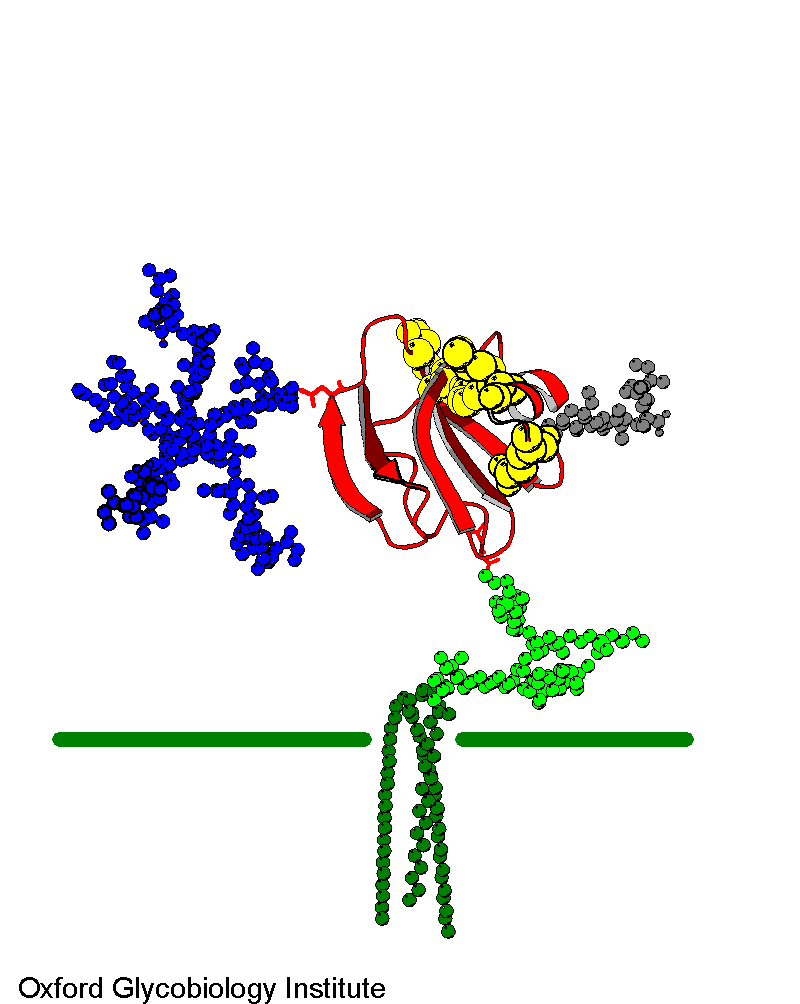
CD59
Molecular model based on the solution NMR structure of soluble CD59 and
the glycosylation analysis. There is a single N-glycosylation site (blue)
and a number of potential O-glycosylation sites, only one of which has been
used. The active site residues are in yellow.
The glycosylation of the complement regulatory protein,
human erythrocytes CD59.
P.M. Rudd, B.P. Morgan, M.R. Wormald, D.J. Harvey,
C.W. van den Berg, S.J. Davis, M.A.J. Ferguson and R.A. Dwek (1997) J. Biol.
Chem., 272, 7229-7244.
Mutational analysis of the active site and antibody
epitopes of the complement-inhibitory glycoprotein, CD59.
D.L. Bodian, S.J. Davis, B.P. Morgan and N.K. Rushmere
(1997) J. Exp. Med., 185, 507-516.
|
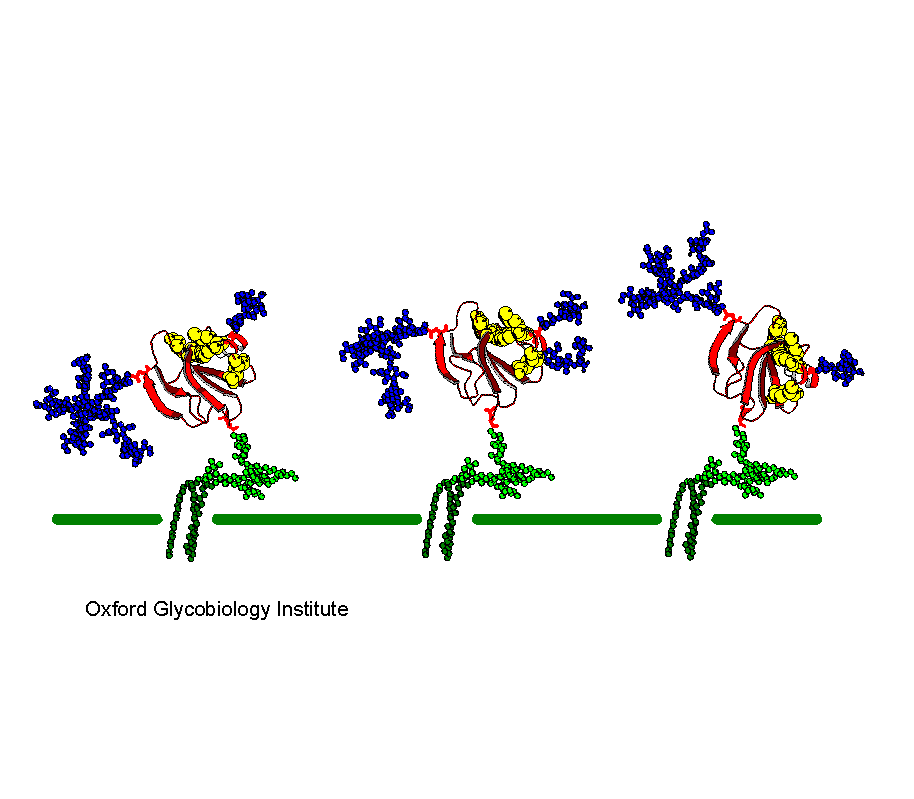
|
CD59
Three different glycoforms of CD59 showing how the presence of glycans
can influence the presentation of the active site residues (yellow) relative
to the membrane.
The glycosylation of the complement regulatory protein,
human erythrocytes CD59.
P.M. Rudd, B.P. Morgan, M.R. Wormald, D.J. Harvey,
C.W. van den Berg, S.J. Davis, M.A.J. Ferguson and R.A. Dwek (1997) J. Biol.
Chem., 272, 7229-7244.
|
|
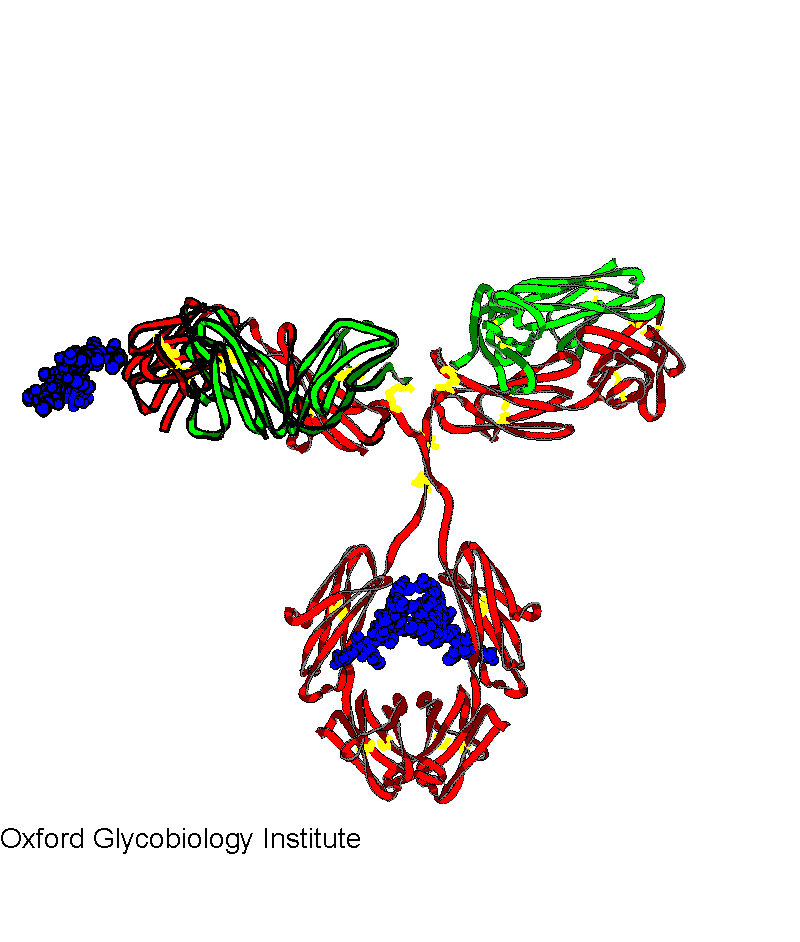
IgG 1
Molecular model based on the crystal structures of IgG Fab and IgG Fc.
Fab glycosylation sites are in the hyper-variable region and occupied approx.
40% of the time.
Glycobiology: The Function of Sugar in the IgG Molecule.
R.A. Dwek, A.C. Lellouch and M.R. Wormald (1995) J.
Anatomy, 187, 279-292.
Variations of oligosaccharide-protein interactions
in immunoglobulin G determine the site-specific glycosylation profiles and
modulate the dynamic motion of the Fc oligosaccharides.
M.R. Wormald, P.M. Rudd, D.J. Harvey, S.-C. Chang,
I.G. Scragg and R.A. Dwek (1997) Biochemistry, 36, 1370-1380.
|
|
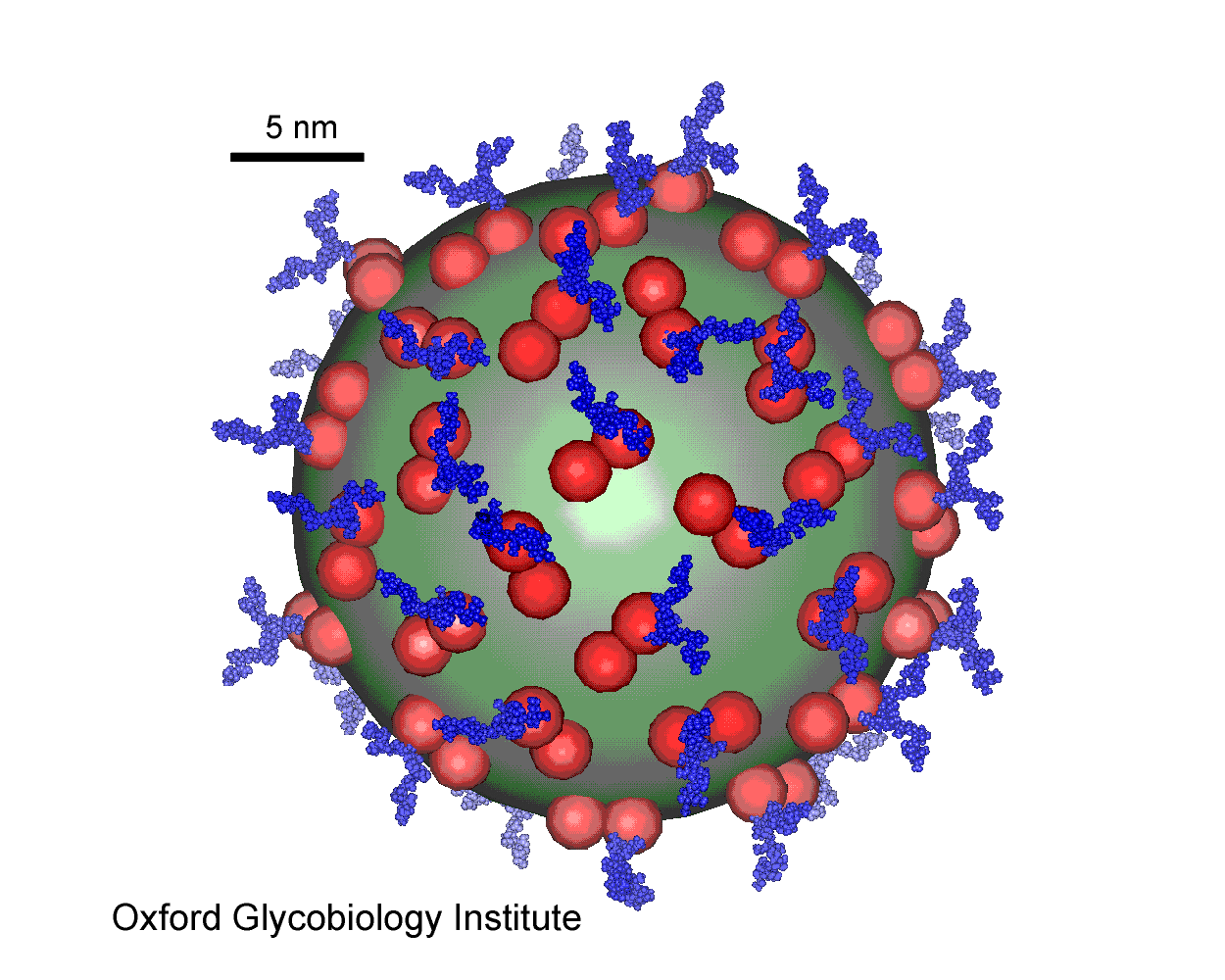
HBV sub-viral particle
Spherical sub-viral particles contain only S-proteins (red). The rod-shaped
viral particles also contain M- and L-proteins.
Treatment of chronic hepadnavirus infection in a
woodchuck animal model with an inhibitor of protein folding and trafficking.
T.M. Block, X.Y. Lu, A.S. Mehta, B.S. Blumberg, B.
Tennant, M. Ebling, B. Korba, D.M. Lansky, G.S. Jacob and R.A. Dwek (1998)
Nature Medicine, 4, 610-614.
a-glucosidase inhibitors
as potential broad based anti-viral agents.
A. Mehta, N. Zitzmann, P.M. Rudd, T.M. Block and R.A.
Dwek (1998) FEBS Letters, 430, 17-22.
|